Filariasis is caused by nematodes (roundworms) that inhabit the lymphatics and subcutaneous tissues. Eight main species infect humans. Three of these are responsible for most of the morbidity due to filariasis: Wuchereria bancrofti and Brugia malayi cause lymphatic filariasis, and Onchocerca volvulus causes onchocerciasis (river blindness). The other five species are Loa loa, Mansonella perstans, M. streptocerca, M. ozzardi, and Brugia timori. (The last species also causes lymphatic filariasis.)
Infective larvae are transmitted by infected biting arthropods during a blood meal. The larvae migrate to the appropriate site of the host's body, where they develop into microfilariae-producing adults. The adults dwell in various human tissues where they can live for several years. The agents of lymphatic filariasis reside in lymphatic vessels and lymph nodes; Onchocerca volvulus in nodules in subcutaneous tissues; Loa loa in subcutaneous tissues, where it migrates actively; Brugia malayi in lymphatics, as with Wuchereria bancrofti; Mansonella streptocerca in the dermis and subcutaneous tissue; Mansonella ozzardi apparently in the subcutaneous tissues; and M. perstans in body cavities and the surrounding tissues. The female worms produce microfilariae which circulate in the blood, except for those of Onchocerca volvulus and Mansonella streptocerca, which are found in the skin, and O. volvulus which invade the eye. The microfilariae infect biting arthropods (mosquitoes for the agents of lymphatic filariasis; blackflies [Simulium] for Onchocerca volvulus; midges for Mansonella perstans and M. streptocerca; and both midges and blackflies for Mansonella ozzardi; and deerflies [Chrysops] for Loa loa). Inside the arthropod, the microfilariae develop in 1 to 2 weeks into infective filariform (third-stage) larvae. During a subsequent blood meal by the insect, the larvae infect the vertebrate host. They migrate to the appropriate site of the host's body, where they develop into adults, a slow process than can require up to 18 months in the case of Onchocerca.
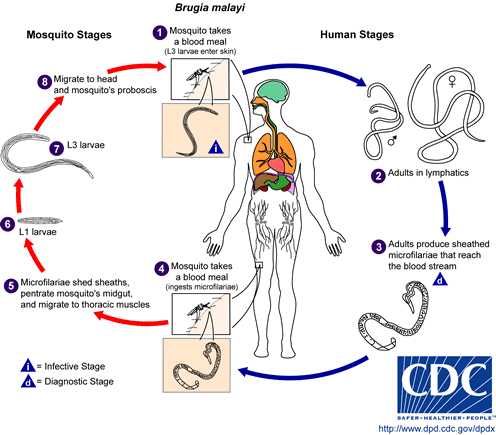
The typical vector for Brugia malayi filariasis are mosquito species from the genera Mansonia and Aedes. During a blood meal, an infected mosquito introduces third-stage filarial larvae onto the skin of the human host, where they penetrate into the bite wound . They develop into adults that commonly reside in the lymphatics . The adult worms resemble those of Wuchereria bancrofti but are smaller. Female worms measure 43 to 55 mm in length by 130 to 170 um in width, and males measure 13 to 23 mm in length by 70 to 80 um in width. Adults produce microfilariae, measuring 177 to 230 um in length and 5 to 7 um in width, which are sheathed and have nocturnal periodicity. The microfilariae migrate into lymph and enter the blood stream reaching the peripheral blood . A mosquito ingests the microfilariae during a blood meal . After ingestion, the microfilariae lose their sheaths and work their way through the wall of the proventriculus and cardiac portion of the midgut to reach the thoracic muscles . There the microfilariae develop into first-stage larvae and subsequently into third-stage larvae . The third-stage larvae migrate through the hemocoel to the mosquito's prosbocis and can infect another human when the mosquito takes a blood meal.
Among the agents of lymphatic filariasis, Wuchereria bancrofti is encountered in tropical areas worldwide; Brugia malayi is limited to Asia; and Brugia timori is restricted to some islands of Indonesia. The agent of river blindness, Onchocerca volvulus, occurs mainly in Africa, with additional foci in Latin America and the Middle East. Among the other species, Loa loa and Mansonella streptocerca are found in Africa; Mansonella perstans occurs in both Africa and South America; and Mansonella ozzardi occurs only ins the Americas, from Mexico south to South America and in the Caribbean.
Lymphatic filariasis most often consists of asymptomatic microfilaremia. Some patients develop lymphatic dysfunction causing lymphedema and elephantiasis (frequently in the lower extremities) and, with Wuchereria bancrofti, hydrocele and scrotal elephantiasis. Episodes of febrile lymphangitis and lymphadenitis may occur. Persons who have newly arrived in disease-endemic areas can develop afebrile episodes of lymphangitis and lymphadenitis. An additional manifestation of filarial infection, mostly in Asia, is pulmonary tropical eosinophilia syndrome, with nocturnal cough and wheezing, fever, and eosinophilia. Onchocerciasis can cause pruritus, dermatitis, onchocercomata (subcutaneous nodules), and lymphadenopathies. The most serious manifestation consists of ocular lesions that can progress to blindness. Loiasis (Loa loa) is often asymptomatic. Episodic angioedema (Calabar swellings) and subconjunctival migration of an adult worm can occur. Infections by Mansonella perstans, while often asymptomatic, can be associated with angioedema, pruritus, fever, headaches, arthralgias, and neurologic manifestations. Mansonella streptocerca can cause skin manifestations including pruritus, papular eruptions and pigmentation changes. Eosinophilia is often prominent in filarial infections. Mansonella ozzardi can cause symptoms that include arthralgias, headaches, fever, pulmonary symptoms, adenopathy, hepatomegaly, and pruritus.
Identification of microfilariae by microscopic examination is the most practical diagnostic procedure. Examination of blood samples will allow identification of microfilariae of Wuchereria bancrofti, Brugia malayi, Brugia timori, Loa loa, Mansonella perstans, and M. ozzardi. It is important to time the blood collection with the known periodicity of the microfilariae. The blood sample can be a thick smear, stained with Giemsa or hematoxylin and eosin. For increased sensitivity, concentration techniques can be used. These include centrifugation of the blood sample lyzed in 2% formalin (Knott's technique), or filtration through a Nucleopore® membrane. Examination of skin snips will identify microfilariae of Onchocerca volvulus and Mansonella streptocerca. Skin snips can be obtained using a corneal-scleral punch, or more simply a scalpel and needle. The sample must be allowed to incubate for 30 minutes to 2 hours in saline or culture medium, and then examined for microfilariae that would have migrated from the tissue to the liquid phase of the specimen.
For more information view the source:Center for Disease Control
Recommended Test: Full GI Panel
Recommended Product:Freedom Cleanse Restore Parasite Cleanse